Abstract
Background: Venetoclax, a selective, potent, orally bioavailable BCL2 inhibitor, has shown synergistic activity with hypomethylating agents in preclinical and clinical studies in high-risk MDS and AML. The primary objective of this study was to describe the safety, effectiveness, and management of this therapy in a real-world scenario.
Methods: High-risk MDS patients receiving 400 mg of oral venetoclax daily for the first 14 days of each 28-day cycle, combined with azacytidine (AZA) at 75 mg/m2 from days 1 to 7 of the 28-day cycle were reported, and retrospectively analyzed. The response rates were evaluated according to IWG-2016. A Log-Rank test (Mantel Cox) and Kaplan-Meier were performed to compare the OS of responders and non-responders.
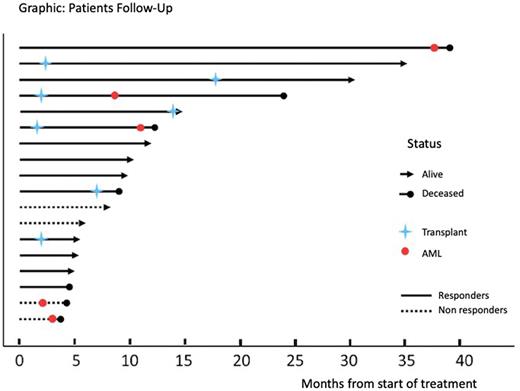
Results: From September 2017 to July 2022, 18 high-risk MDS patients under venetoclax and azacytidine were retrospectively reported. This Latin-American multicenter study showed a median age of 63 (R 37-81); 61% male; 16% median blasts at diagnosis (R 0-19); an MDS subtype of MDS_EB (72%); therapy-related MDS (11%); IPSS-R > 4.5 (83%); and high, and very high-risk cytogenetic subgroups (53%) at diagnosis. Fifteen patients (83%) were naïve for hypomethylating agents. With a median cycle of 4.5 (R 1-39), the overall response rate was 78% (Complete Remission 33%, Partial Remission 17%, medular Complete Remission 17%, and Hematology Improvement 11%) and 22% for non-responders (Stable Disease and Progressive Disease). A total of 7 (39%) patients underwent transplantation, and the median number of cycles to reach the transplant was 1 (R 1-5). The estimated median overall survival (months) for responders versus non-responders was 39.1 vs 4.5 respectively (p=0.024). Five patients (28%) progressed to AML, and there was no correlation with the type and depth of the response achieved (Graphic). The most common treatment-emergent adverse events were neutropenia and infectious diseases (22%). Dose reductions or adjustments to azoles were necessary in 10 patients (56%). There were 7 deaths, 6 of which were related to MDS: infections (3), hemorrhage (1), and AML (2). One patient in Complete Remission died because of Covid-19.
Conclusion: Combination therapy of venetoclax and azacytidine in high-risk MDS patients demonstrated a tolerable safety profile, a frequent need of dose adjustments, a high overall response rate (78%), a statistically significant longer overall survival of responders (p=0.024) and a high efficacy as bridge-therapy to transplant. Further experiences are warranted.
Disclosures
Iastrebner:BMS: Honoraria, Other: Advisory Board, Speakers Bureau; Abbvie: Honoraria, Other: Advisory Board, Speakers Bureau. Gomez-De Leon:AbbVie: Honoraria, Other: advisory board.
OffLabel Disclosure:
The combination of Venetoclax + Azacytidine therapy is an off-label therapy in MDS.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal